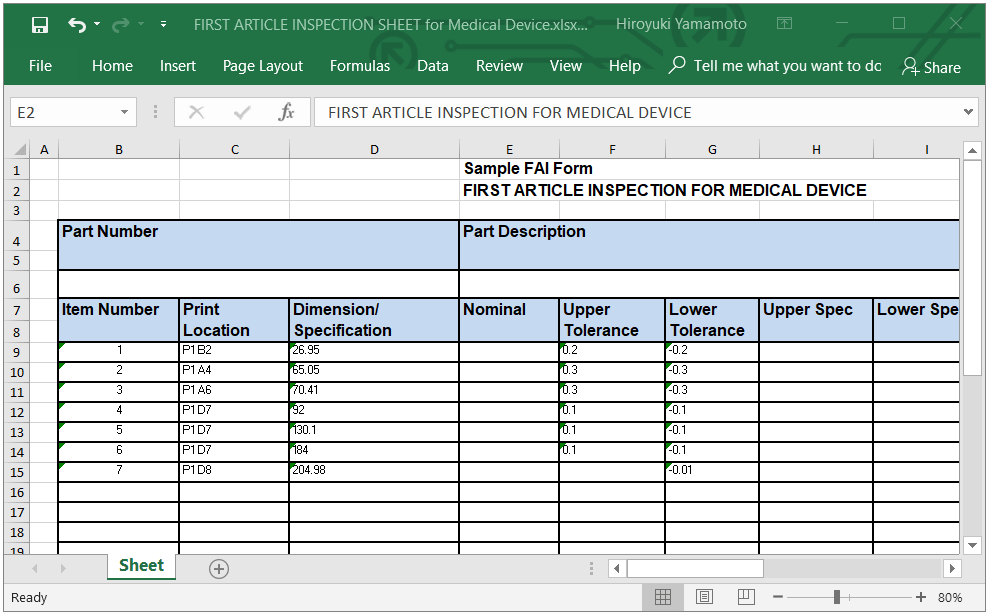
ISO 13845 First Article Inspection - Medical Device FAI
Download Free Sample Medical Device FAI form (Excel file with unlocked sheets and cells).
In regards to the inspection of medical devices, there are two standards, ISO 13845 and FDA 21 CFR Part 820.
In similar to the AS9102, a ballooned drawing and an inspection form are required to verify that the part has been manufactured correctly.
Please download our FREE first article inspection sample form for medical devices to complete your FAI process more easily and efficiently.
Download Free FAI EXCEL Form
Download Free FAI PDF Form
If you do not want to complete these FAI forms by hand, we would like to suggest the free trial of our QA-CAD software,
because QA-CAD creates FAI report quickly and accurately.
QA-CAD supports PDF, AutoCAD drawing, Microstation DGN, TIFF, JPG, BMP and other raster image formats.
Start QA-CAD Software Free Trial